Source: https://www.gemsociety.org/article/are-diamonds-really-rare/
Although diamond is our most popular gemstone, this hasn’t always been the case. Only in the last century did diamonds become readily available. Prior to that, ruby and sapphire were the most popular gems, especially for engagement rings.

Diamonds and the De Beers Corporation
Diamonds ascended in the public imagination primarily due to the De Beers corporation. They set up the first large-scale diamond mines in South Africa. Then, they began one of the most successful advertising campaigns in history, convincing consumers that engagement rings should always have a diamond.

Diamond Advertising Campaigns
With proper encouragement, the movie industry displayed its most glamorous actresses, draped in diamonds. As a result, diamonds soon became a top status symbol for the rich and famous. This peaked perhaps with Marilyn Monroe’s performance of the song, Diamonds are a Girl’s Best Friend, in the 1953 film, Gentlemen Prefer Blondes.
Even after winning the admiration of consumers, De Beers continued their advertising. With the discovery of diamonds in the (now former) Soviet Union, they created a new campaign to sell anniversary bands. These made good use of the small but nice-quality diamonds this find produced.
Cornering the Market
De Beers did wonderful things for the diamond industry. However, not everything about De Beers is nice. As diamonds were discovered in other parts of Africa and South America, De Beers gained control of the rough diamond supply. Allegedly, the tactics used to gain control included murder and kidnapping.
De Beers maintained a monopolistic hold over the diamond market for several decades, controlling 75-85% of the diamond rough supply. They carefully released only enough rough diamonds to satisfy then-current demand, while continually adjusting the degree of rough diamond availability. Of course, this made prices escalate and reinforced the perception of diamond’s rarity. De Beers actually mined considerably more rough diamonds than they sold. They maintained a large warehouse of uncut diamonds in London. As a result, they weren’t allowed to do business in the United States and a few other countries.
New Discoveries and Developments
In the last two decades of the 20th century, things began to change.
Satellite technology, originally designed to find likely oil reserves, also showed the geology likely to hold diamonds. As a result, new discoveries began to multiply. For example, Australia became one of the first developed nations to discover major diamond resources. De Beers made a deal with them to distribute all the rough, except for the very rare pink diamonds. In 1996, Australia ended its arrangement with De Beers.
De Beers also made a deal with the Soviet Union to distribute their rough diamonds. However, shortly after the Soviet breakup, the Russians let their contract expire and began to sell the diamonds themselves.
In 1999, the De Beers London stockpile was valued at $5.2 billion, but they agreed to stop stockpiling diamonds in 2000. In 2004, De Beers agreed to plead guilty to criminal price fixing before a U.S. federal court. This allowed them to once again sell diamonds in the U.S. (In 2012, the U.S. Supreme Court refused to consider an appeal by De Beers against a class action settlement regarding various unfair business practices).

What Does the Future Hold?
With explorations of several new sites, more diamond deposits will likely be found in the near future. De Beers still controls approximately 35-40% of the diamond rough supply. So far, the other suppliers have been content to sell at the same prices as De Beers. However, if the law of supply and demand ever catches up to the diamond market, prices will likely drop considerably. What would happen next is difficult to tell. De Beers has a large inventory of uncut diamonds and holds an excellent position for a price war.

Diamond Myths and Misconceptions
Now that you know some of the history behind the rise of diamond’s popularity, let’s debunk some popular diamond myths.
MYTH: Diamonds are Rare
Diamonds are the hardest material found on earth. They resist scratching better than anything else. Other than that, they hold no unique distinctions. All gem-quality materials are rare. They compose just a tiny fraction of the Earth. However, diamonds actually number among the most common gems. Ask yourself this: “How many people do you know who own at least one diamond?” Now, ask this question about other gems, like rubies, sapphires, or emeralds.
While we have much to learn about the Earth’s interior, our current knowledge of gem formation indicates that diamonds are likely the most common gem in nature.
Outside the confines of the Earth, diamonds are still common. A recent discovery indicates that some stars collapse on themselves, creating giant diamond crystals. In the constellation Centaurus, there lies a white dwarf that has crystallized into a diamond about 2,500 miles in diameter and weighing 10 billion, trillion, trillion carats.
MYTH: Diamonds are the Most Valuable Gem
You can’t judge one gem species as the most valuable. To compare gem value, you have to evaluate gems according to size and quality. The table below shows values for top quality gems of different sizes. However, please note that pure red rubies are so rare there is no trade data available. The ruby prices listed are for Burmese rubies.
| Species | 1 carat | 1-5 carat | 5+ carats |
| Diamond | $4,300/ct | $13,600/ct | $44,500/ct |
| Ruby | $5,050/ct | $9,500/ct | $100,000/ct |
| Emerald | $5,470/ct | $9,030/ct | $23,000/ct |
| Sapphire | $10,000/ct | $16,000/ct | $34,000/ct |
| Alexandrite | $3,600/ct | $15,000/ct | $70,000/ct |
As you can see, while costly, diamonds aren’t the most expensive gem in any size. If you compare diamonds with gems based on other qualities, you’ll get similar results. If you’re looking to invest in gems, read our introduction to gemstone investing and our article on the inner workings of the gem trade.

MYTH: Diamonds are Precious Stones
“Precious” means valuable. In the 18th century, a French jeweler began describing gems as either precious or semiprecious stones. While you’ll find these categories still used in merchandising, professional gemologists frown upon these terms. This distinction has no real meaning. For example, garnet gems traditionally fall into the semiprecious category. Nevertheless, tsavorite garnets, for example, have sold for as much as $10,000 per carat. That seems pretty “precious” to me!

On the other hand, diamonds only have very high value in their better grades and medium to large sizes. You can find small, low-quality diamonds in quantity for just $1 a piece. On eBay, you can find many diamonds under $20. These are far from precious.
MYTH: Diamonds are the Most Brilliant Gemstone
The faceting and the refractive index (RI) of a gem determine its brilliance. That means how much light it reflects back to the viewer. Diamonds do have a very high RI of 2.41. If properly cut, diamonds have the potential for exceptional brilliance. However, that pales in comparison to rutile’s RI of 2.90. Not counting synthetics, at least 8 minerals have a higher RI than diamond.
MYTH: A Person can Make a lot of Money Selling Diamonds
Due to the proliferation of the Internet and the establishment of well-accepted diamond grading standards, margins on cut diamonds have become extremely thin. Not infrequently, diamond dealers make gross margins inside of 5%. Compare that to virtually any other industry. You won’t think selling diamonds makes a great business opportunity anymore.
MYTH: Diamonds Have More “Fire” Than any Other Gemstone
Diamonds are known for their “fire” or dispersion. This refers to their ability to separate white light into the colors of the rainbow. In fact, diamond does have quite a high dispersion value of 0.044. However, that’s a far cry from rutile, again, with a dispersion of 0.280. In fact, synthetic rutile stones, known as “Titania,” used to be sold as imitation diamonds. However, they showed too much dispersion to make passable diamonds!

What is a Diamond?
Gemologically speaking, diamond is a mineral with a chemical formula of C (carbon) that crystallizes in the isometric system. By comparison, the mineral graphite also has the chemical formula of C. However, it crystallizes in the hexagonal system and has very different properties.
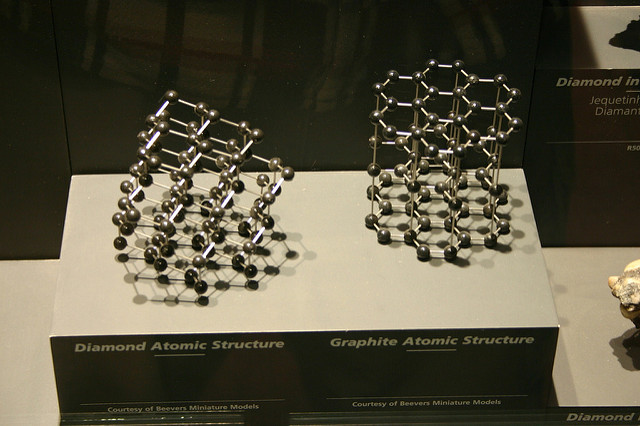
As isometric crystals, diamonds have a single RI, so they don’t show pleochroism or birefringence. They have a specific gravity or density of 3.51 to 3.53, a bit more than average for gemstones. These properties can help distinguish real diamonds from some of their look-alikes.
As noted previously, diamonds have a hardness of 10, the highest of any material in nature. Scientists have created harder substances, but they’re extremely brittle and have no practical use. (“Hardness” is just one measure of a material’s durability). If researchers ever find a harder substance that doesn’t break down so quickly, it will greatly reduce the time needed to cut diamonds.
While diamonds occur in nature, laboratories can also synthesize them. Lab-made diamonds serve primarily as abrasives. However, they’re quickly making their way into the jewelry industry. For more information, see our FAQ about lab-created diamonds and article on identifying synthetic diamonds.
If you’d like to learn more about gemology, start with this introduction.


