Source: https://www.gia.edu/ruby-care-cleaning

Hardness and toughness
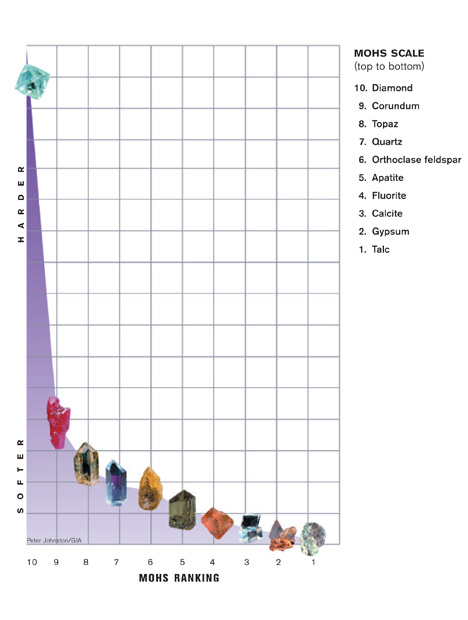
Gem and mineral hardness is measured on the Mohs scale. The numbers are based on the relative ease or difficulty with which one mineral can be scratched by another. But the Mohs scale is deceptive. The steps between the minerals are not evenly spaced. For example, diamond is only one number away, but it’s many times harder than gems in the corundum family.
Corundum (ruby and sapphire) is relatively hard—9 on the Mohs scale. It has excellent toughness and no cleavage, which is a tendency to break when struck. This makes it a great choice for rings and other mountings subject to daily wear.
Ruby ranks 9 on the Mohs hardness scale, so it’s an effective jewelry stone.
Stability
Corundum is stable under normal wearing conditions, which means it’s resistant to the effects of heat, light, and common chemicals. Boric acid powder will etch the surface of even untreated stones. Fracture-filled, cavity-filled, and dyed stones can be damaged by even mild acids like lemon juice.
Cleaning
Warm soapy water is always safe. Ultrasonic and steam cleaners are usually safe for untreated, heat-treated, and lattice diffusion treated stones. Fracture-filled, cavity-filled, or dyed material should only be cleaned with a damp cloth.
Treatment and durability considerations
Untreated ruby and even heat-treated ruby are very durable. Stones that have undergone lattice diffusion treatment have varying degrees of treated-color penetration. In some stones, the treated color penetrates the entire stone, while others have very shallow treated-color penetration. For stones with shallow color penetration, surface damage or re-cutting can remove color.
Today’s fracture-filled stones have surface-reaching fractures filled primarily with a high-lead content glass. There are large numbers of these treated rubies in the market and they require greater care than untreated, heat-treated, or lattice diffusion treated ruby. The glass can be damaged through contact with a variety of chemicals. Even relatively mild substances like lemon juice can cause changes in the high-lead content glass.

